Is Bromine A Diatomic . instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. Bromine is one of the seven diatomic molecules. some elements exist naturally as molecules.
from www.numerade.com
Bromine is one of the seven diatomic molecules. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. some elements exist naturally as molecules. like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond.
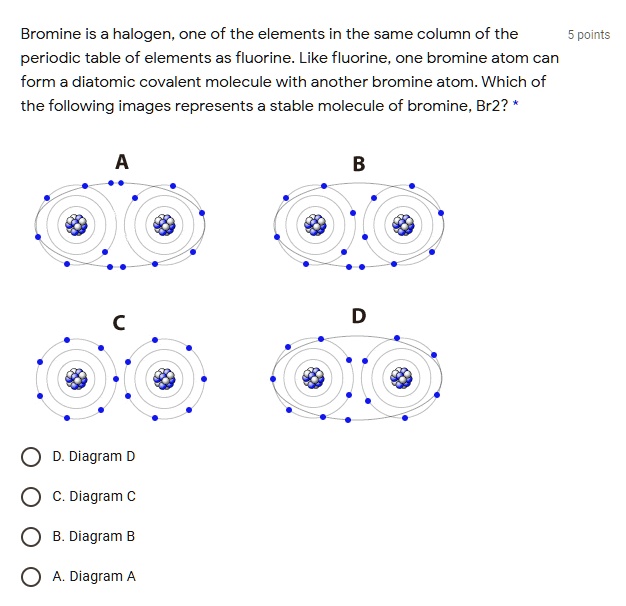
Bromine is a halogen; one of the elements in the same column of the
Is Bromine A Diatomic diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. some elements exist naturally as molecules. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. Bromine is one of the seven diatomic molecules. like the other halogens, bromine exists as diatomic molecules in all aggregation states. instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. diatomic elements are pure elements that form molecules consisting of two atoms bonded together.
From thechemistrynotes.com
Diatomic Elements Important 7 Elements properties formation Is Bromine A Diatomic some elements exist naturally as molecules. instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic elements are pure elements that form. Is Bromine A Diatomic.
From www.anyrgb.com
Pblock, homonuclear Molecule, bromine Dioxide, iodine127, diatomic Is Bromine A Diatomic like the other halogens, bromine exists as diatomic molecules in all aggregation states. Bromine is one of the seven diatomic molecules. some elements exist naturally as molecules. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. instead, they. Is Bromine A Diatomic.
From geteducationbee.com
Diatomic Elements Best Definition, Example & More Get Education Bee Is Bromine A Diatomic Bromine is one of the seven diatomic molecules. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. some elements exist naturally as molecules. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. like the other halogens, bromine exists as diatomic molecules in all aggregation. Is Bromine A Diatomic.
From www.numerade.com
SOLVEDBromine is a diatomic molecule, and it has two isotopes, ^79 Br Is Bromine A Diatomic About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. Bromine is one of the seven diatomic molecules. like the other halogens, bromine exists as diatomic molecules in all aggregation states. instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. diatomic elements are pure elements that. Is Bromine A Diatomic.
From pablochemgroup5.blogspot.com
Periodic Table (By Carl Andrew Pimentel) Is Bromine A Diatomic instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. Bromine is one of the seven diatomic molecules. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. diatomic elements are pure elements that form molecules consisting of two atoms bonded together.. Is Bromine A Diatomic.
From cebuzlwv.blob.core.windows.net
Diatomic Bromine Uses at Joseph Carter blog Is Bromine A Diatomic some elements exist naturally as molecules. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. like the other halogens, bromine exists as diatomic molecules in all aggregation states. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. diatomic elements are pure elements that form molecules consisting of. Is Bromine A Diatomic.
From www.chegg.com
Solved 1. Bromine is a diatomic molecule with a boiling Is Bromine A Diatomic diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Bromine is one of the seven diatomic molecules. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form.. Is Bromine A Diatomic.
From www.numerade.com
Bromine is a halogen; one of the elements in the same column of the Is Bromine A Diatomic diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. like the other halogens, bromine exists as diatomic molecules in all aggregation states. Bromine is one of the seven diatomic molecules. diatomic elements are pure elements that form molecules consisting. Is Bromine A Diatomic.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Is Bromine A Diatomic like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. some elements exist naturally as molecules. Bromine is one of the seven diatomic molecules. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. instead, they. Is Bromine A Diatomic.
From exolwdsjz.blob.core.windows.net
A Bromine Atom Has An Atomic Number Of 35 at Oscar Broom blog Is Bromine A Diatomic About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. some elements exist naturally as molecules. Bromine is one of the seven diatomic molecules. diatomic elements are pure elements that form molecules consisting of two atoms bonded. Is Bromine A Diatomic.
From exouzacfu.blob.core.windows.net
Bromine Of Formula at Delores Humphrey blog Is Bromine A Diatomic like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. Bromine is one of the seven diatomic molecules. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. About 3.41 grams (0.12 ounce) of. Is Bromine A Diatomic.
From www.chegg.com
Solved Bromine is a diatomic molecule, and it has two Is Bromine A Diatomic instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. Bromine is one of the seven diatomic molecules. some elements exist. Is Bromine A Diatomic.
From www.researchgate.net
a Formation of a bromine molecule from dissociative adsorption of two Is Bromine A Diatomic Bromine is one of the seven diatomic molecules. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic elements are pure elements that form molecules consisting. Is Bromine A Diatomic.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.46 Understand How to Use DotandCross Is Bromine A Diatomic diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. Bromine is one of the seven diatomic molecules. instead, they are in the. Is Bromine A Diatomic.
From www.coursehero.com
[Solved] . Written response question 1 (Q1) Bromine is a diatomic Is Bromine A Diatomic some elements exist naturally as molecules. Bromine is one of the seven diatomic molecules. like the other halogens, bromine exists as diatomic molecules in all aggregation states. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. instead, they are in the form of f 2 or o 2, the diatomic element or molecular element form.. Is Bromine A Diatomic.
From brainly.com
Bromine is a halogen, one of the elements in the same column of the Is Bromine A Diatomic like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. some elements exist naturally as molecules. About 3.41 grams (0.12 ounce) of bromine. Is Bromine A Diatomic.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Is Bromine A Diatomic About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters. like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. instead, they are in the form of f 2 or o 2, the diatomic element or molecular. Is Bromine A Diatomic.
From cebuzlwv.blob.core.windows.net
Diatomic Bromine Uses at Joseph Carter blog Is Bromine A Diatomic diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. like the other halogens, bromine exists as diatomic molecules in all aggregation states. diatomic elements are pure elements that form molecules consisting of two atoms bonded together. some elements exist naturally as molecules. Bromine is one of the seven diatomic. Is Bromine A Diatomic.